Now that the Pfizer, Moderna, and Janssen COVID-19 vaccines received emergency use authorization, supply will hopefully increase and an end to the pandemic will eventually ensue. But what should you and your employees know about these vaccines? As the market leader in COVID-19 solutions, we answer some of the questions you may have.

What to Expect
Which vaccine should I get?
Whichever one is available to you once you’re eligible. All of the authorized COVID-19 vaccines have been shown to significantly reduce the risk of serious illness from COVID-19. If you have a history of allergic reactions, are pregnant, or are immunocompromised, be sure to talk to your medical provider before receiving a vaccine.
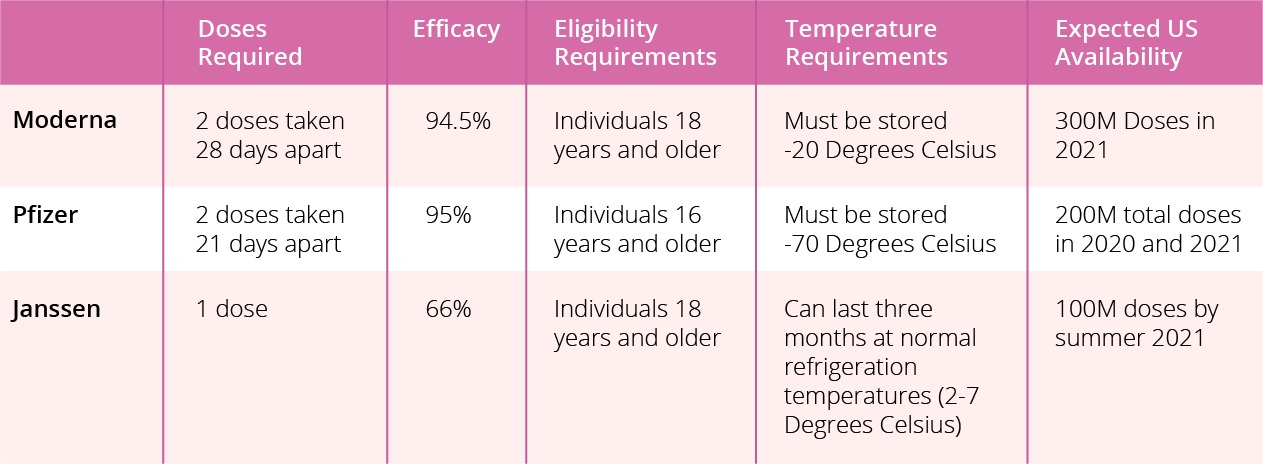
What are the key differences between the vaccines?
What are the side effects?
The majority of participants in all three vaccine studies have reported no serious side effects. However, some participants have reported fatigue, headache, fever, and soreness at the injection site. These mild side effects are common for other vaccines too, and they only last a day or two. If you experience similar side effects, you should not panic; you should interpret it as a sign that your body is starting to produce an immune response. If you want extra peace of mind, contact your medical provider.
Additionally, consult your provider before getting vaccinated if you have a history of severe allergic reactions to vaccines. After receiving the vaccine, you will be asked to stay for 15 minutes so a provider can monitor you for signs of an allergic reaction, which are extremely rare but treatable. If you have a history of severe allergies, you will be asked to stay for 30 minutes after the appointment to be extra safe.
How many doses are required for each vaccine?
The Janssen vaccine requires one shot. Pfizer’s vaccine requires two shots taken 21 days apart, and Moderna’s vaccine requires two shots taken 28 days apart. Remember, both shots of the Pfizer and Moderna vaccine must come from the same manufacturer; they are not interchangeable.
If you only take one dose of a vaccine that requires two, you will not develop full immunity to COVID-19. Therefore, not showing up to your second appointment could undermine vaccination efforts. Although it is unlikely, the novel coronavirus could even become vaccine-resistant if millions of people only take one dose of a vaccine that requires a booster.
How much will the COVID-19 vaccine cost?
Federal Health officials have said that the COVID-19 vaccine will be free for anyone who chooses to receive it. You will not receive a bill or need to worry about receiving the vaccine from a provider in your insurance network.
Eligibility
What populations are eligible for vaccination under the EUA?
Pfizer’s emergency use authorization includes individuals 16 years and older, while Moderna’s and Janssen’s emergency use authorizations include individuals 18 years and older.
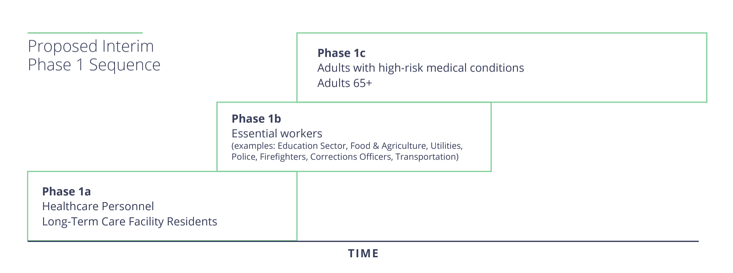
Who will receive the vaccines first?
Although the CDC outlined a recommendation for who should receive the vaccine first, each state’s top health officials and governors can alter the distribution plan based on their local needs. As outlined in the graphic below, healthcare personnel and long-term care facilities are generally prioritized to receive the vaccines first. Note that states do not need to vaccinate everyone in each priority group before moving to the next group. To learn more about the eligibility criteria in your local area, check out our state-by-state vaccination guide.
Should I still get the vaccine if I’ve already had COVID-19 or tested positive for COVID-19 antibodies?
Getting COVID-19 might offer some natural protection or immunity from reinfection with the virus that causes COVID-19. However, it’s not clear how long this protection lasts. Because reinfection is possible and can cause severe medical complications, it’s recommended that people who have already had COVID-19 still get a COVID-19 vaccine.
What populations were not included in Pfizer, Moderna, and Janssen’s clinical trials?
We do not yet have trial data about some subsets of the population such as pregnant patients and children under 12 years of age for the Pfizer vaccine. Similarly, Moderna and Janssen’s vaccine trials do not yet have data for pregnant women and children under 18 years of age.
It is important to note that the current lack of data does not indicate increased suspicion from researchers and physicians about adverse effects in these populations. In fact, pregnant patients routinely receive vaccinations for illnesses such as the flu during their pregnancies. If you are pregnant and want extra guidance, the American College of Obstetricians and Gynecologists (ACOG) suggests consulting your medical provider before getting vaccinated against COVID-19.
Understanding the Vaccines
Can I get COVID-19 from the vaccines?
No. The Moderna and Pfizer vaccines use non-infectious genetic material, called messenger RNA, which cannot cause a COVID-19 infection. The Janssen vaccine also cannot cause a COVID-19 infection because the virus used in the vaccine has been modified so it cannot replicate.
Will the vaccines cure coronavirus in those already infected?
No. As with most vaccines, a COVID-19 vaccine will only help prevent symptom development in people who are exposed to the virus. However, other medicines are being tested and developed to treat patients currently infected with COVID-19.
If I’m young, healthy, and not at risk for COVID-19, why would I need a vaccine if I’d probably recover from the virus?
The danger of getting sick with COVID-19 far outweighs the risks of getting the vaccine; vaccination is safe, and it will prevent you from developing symptoms and experiencing serious complications caused by the virus. Although it is rarer for young, healthy individuals to die from COVID-19, no one can guarantee how your body will respond to the virus. Moreover, younger populations who have been infected with COVID-19 have reported chronic issues, and you may be unaware of certain underlying conditions that may complicate your recovery.
Healthy individuals also make up a large portion of the population, so if all healthy individuals abstain from getting vaccinated, we will never reach herd immunity and end the pandemic. Studies are being conducted to see if these vaccines effectively stop the spread of the novel coronavirus in addition to preventing disease development. These studies may provide definitive evidence that the vaccine not only protects you as the recipient, but also your loved ones.
After Vaccination
Now that vaccines are available, do we still need to wear masks and socially distance?
Yes. These measures are still necessary to slow the spread of COVID-19, especially now. It will take months before the vaccines are widely available, and we need to continue to take extra precautions until everyone can get vaccinated.
It’s also unknown whether vaccinated individuals are less likely to transmit the virus. If these people can unknowingly spread COVID-19, masks and social distancing will continue to be critical tools in protecting others. Additionally, active monitoring of symptoms and exposures and accessible COVID-19 testing are still important for mitigating the spread of the virus.
If you notice COVID-19 symptoms after getting vaccinated, should you isolate?
Yes. Because it takes a few weeks to build up immunity after receiving the vaccines, it is possible to become infected with COVID-19 right before (or after) vaccination. This could happen because the vaccine has not had sufficient time in your body to provide protection or because you have not had both doses of the Pfizer or Moderna vaccine. If this occurs, you should contact your provider, explain your symptoms, and isolate accordingly.
–
While this news is exciting, we still have months before the vaccines will be widely available. And even when the vaccines are accessible to everyone who wants it, majority of the population needs to get vaccinated in order for the pandemic to end. Until then, we need to use other tools to help reduce the spread of the virus. For more information on COVID-19 vaccines, visit our blog post about emergency use authorizations.
Disclaimer: This information is based on current resources available and is subject to change. This document and its contents are provided for informational purposes only, and not intended to be, and should not be understood or treated as, a substitute for professional medical advice around COVID-19, its risks or symptoms, or to take the place of any local, state and national laws and guidelines around COVID-19. Always seek the advice of a physician or other qualified health provider with any questions you may have regarding a medical condition.
This post was updated on 3.1.21